Exhibit A, the phase diagram for a copper- tin alloy, usually known as bronze (Stolen from wikipedia or wherever):

Along the bottom is the weight % tin, i.e. if the object weighs 1kg, and is 50% tin, there is 500g of tin. Sure, that doesn’t seem as scientific as going by mols, but then a lot of metallurgy harks back to before it was properly integrated into modern chemistry.
Anyway, up the left hand side you can see the temperature. Clearly, 100% copper will melt at 1084.87 degrees C, and pure tin will melt at 231.97 degrees C.
The fun bit is what happens in-between. If you think of a bronze tool or gun, it will be around 10% tin, meaning it will melt at around 1010 degrees C, which when you think it also needs a 100 to 200 degrees superheat to ensure it flows into the mould means you are aiming at 1200C, which is quite hot.
On the other hand if you are making a bell with say 22% tin, it will melt at around 900C, which is lower and a bit easier to reach. Not that much easier, mind you, but believe me, every little bit of help with temperature, whether adding more or taking away the need for it, helps.
You can also get these diagrams for alloys of 3 metals, and I expect that nowadays with the help of computers we could plot for more complex alloys as well. Medieval castings often contained 4 or 5 elements in various proportions, with copper being the biggest constituent. Others included tin, lead, zinc, antimony. Here is a 3 metal alloy phase diagram for leaded bronze:
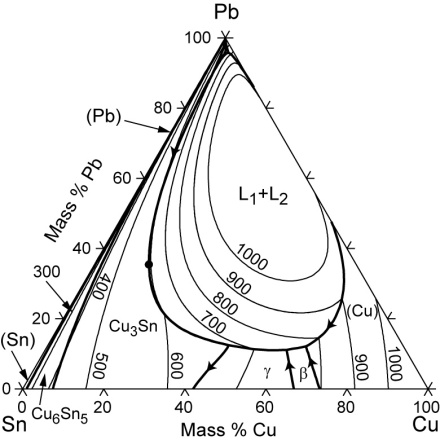
Now, you see it has three sides, for each metal, and the lines on it are isotherms, delineating the area where a specific temperature is required to melt the metal.
What you can see is that adding lots of tin or lead to alloy with the copper reduces the melting point of the alloy, although say if you add 15% Pb and 5% Sn it’ll be a bit under 1000C, whereas if you add 20% Sn it’ll be well under 1,000C, more like 910 or so, so the repeated insistence by archaeometallurgists and others that lead was used in historic castings because it lowers the melting point I find to be trivially true, but certainly not the whole story of why you would use it.
The other bit metallurgical reason I keep reading in books and papers as to why lead is used because it makes the liquid alloy more fluid so it can more easily flow into all the little bits of the casting.
Of course I accepted it at the time, but now am wondering. Exactly why this should happen, I am not sure, it’s something to do with chemistry, and either you can pay me lots of money to experimentally verify the chemistry, or I can find the information in a textbook somewhere, but so far I haven’t.
One website (http://pages.ucsd.edu/~dkjordan/arch/metallurgy.html) says “The lead in the alloy does not become part of its crystalline structure, increasing the fluidity of compound when it is in its molten state.” which doesn’t make sense because by definition the liquid alloy does not have a crystal structure.
Maybe, as many people point out, the historical use of lead is just because of price, it being cheaper than other constituents. It was certainly used for that reason in later medieval England, with some copper alloy cauldrons having their legs melt in the fire because they contained too much lead. I am sure that it was a large part of the reason why it was used.
(This diagram also shows why I have trouble with making an alchemical alloy called Molybdochalkon which is made of lead and copper, because if you look along the top right side between copper and lead, even a 50/50 mix of the two will have a melting point of just under 1000C, at which temperature the lead will evaporate somewhat and it’s all a bit hard to do.)
Further searching online produced the following information:
The famous Dungworth and Nicholas paper on Caldarium, an alloy used for making bronze cauldrons etc in medieval England, can be found here:
Unfortunately even it makes the same claim re. Lead lowering melting point and reducing viscosity, without a reference for it.
A google search finds this paper:
http://www.sciencedirect.com/science/article/pii/0305440377900589
by Craddock, who knew what he was doing, and it apparently mentions the same improved flow by adding lead.
Whilst researching this post I found this interesting round up of information re. Bronze age to Classical bronze objects in Greece/ neart east:
Annoyingly it claims, without reference, that leaded bronze melts at a lower temp and flows better in the intro, then on page 31 it makes the same statement, but references it to a 1994 PhD dissertation from some obscure American university, which is just silly.
Apparently this states that up to 2% of Pb improves fluidity:
http://onlinelibrary.wiley.com/doi/10.1111/j.1475-4754.1992.tb00480.x/abstract
As does another paper by Craddock.
So, after all sorts of looking around, I realise that 1) my library is woefully short of books on metallurgy, and 2) nobody seems to know why adding lead makes bronze more fluid for casting. Or rather, the internet is still not the great source of knowledge and information that people would like it to be. Some source book or paper somewhere has to say why people think up to 2% of Pb added to a copper alloy melt will help it flow, but I have no idea where it is. Any suggestions gratefully accepted.

Pingback: Wheel’s Gazette: Year 2, Vol. #30 | Whewell's Ghost
I had heard that bells were cast from bronze from ancient bronze sculptures and the like. Bell metal is high in tin, which could be added. But also low in lead. Did they have the ability to remove lead from Bronze alloys in medieval times?
LikeLike
IIRC, they removed lead from some stuff by oxidation and evaporation, but that would still lose you the tin through vaporisation, and oxidise copper. It’s not something that would be easily done.
I’d be really wary of any claims that later medieval foundrymen were using ancient statues as sources though, since over a thousand years lots of things happened to them. Besides, do we know that they used leaded bronze back then anyway?
LikeLike
Roman statues had quite high lead contents. One Italian paper says 10-30% Pb. Greek and Etruscan were 0-10% (roughly). But bell metal is shown as at about 1% lead. Tin makes the alloy harder and more brittle, good for bells and mirrors, but not much else. Lead presumably makes it softer. So how could they have melted down, say, roman statues, to make bells unless they had a way to purify out most of the lead? Adding tin would have been no problem, but taking something out of an alloy seems a whole lot more difficult. If lead has a lower melting point than copper and tin perhaps they knew how to heat the alloy and have the lead run off. I don’t know, this is a new area for me.
LikeLike
I guess my guess was right, you can remove lead from copper by heating it until the lead melts, and starts to come out in droplets: https://en.wikipedia.org/wiki/Liquation
LikeLike
Ah, but it depends which bells and when. I’m pretty sure that by late medieval period they were very careful about their bells and didn’t let lead anywhere near it.
I don’t have any sources for medieval Italy, but certainly in England, Brownsword was writing about bell metal being tin and copper with no other alloying agents.
Moreover there are major problems with any pre-modern analyses, because of the problems with sampling of copper alloy artefacts, and the relatively primitive analytical methods themselves.
The major problem is that your question presupposes they melted old high lead statues down to make bells, but that might not have been the case at all, or else took place in the early medieval period when they were just not as concerned with the quality of the bells that were made.
Agricola’s De RE Metallica explains liquation cakes, which involves mixing lead with copper that has some silver in it, then heating the alloy so that the lead, with silver, liquifies and pours away down a trough. The problem there is that just by melting point the expensive tin will come out too, so then you have to try and separate tin and lead, which is not something I’ve ever heard of anyone doing. Tin and lead mix together well, something that Biringuccio notes, and was of course exploited to make the commercially valuable alloy pewter.
What I think is more likely is that most of the bell would be new, fairly clean metal, but some older stuff would be added. Trevor Jennings notes a number of instances of bells being made from copper, tin and all sorts of scrap, pewter and brass and other stuff, although mostly in the post-medieval period. Unfortunately a lot of Jennings data is old and I can’t recall where I’ve read about modern analyses of bell metal.
LikeLike